Presentation at a workshop on Clinical Trials – Clinical Research Program held at the King Fahad Medical City, Riyadh on 27 June 2021 at 10.00 am. By: Professor Omar Hasan Kasule MB ChB (MUK), MPH (Harvard), DrPH (Harvard) Professor of Epidemiology and Bioethics, King Fahad Medical City
NATURAL EXPERIMENTS· Experimental studies involve deliberate human action or intervention whose outcome is then observed. Their objective is to establish the definitive causal relation.
· Examples of experimental studies involving humans: clinical therapeutic trial, community intervention study to assess vaccines, community intervention to assess preventive measures, etc.
· Main characteristics of an experimental design are: random selection, random assignment, manipulation of independent variables by the experimenter, and control of all other variables by the experimenter without manipulating the outcome (dependent variable).
· Experimental studies can be natural experiments or true experiments.
TRUE EXPERIMENTS· Natural experiments are not designed by humans. Humans only observe and benefit from their results.
· Examples of natural experiments.
· Snow's study of cholera in London: he found cholera to be related to the source of the water supply.
· In the 1950s air pollution episodes leading to mortality in London and Los Angeles.
· Increased death and leukemia in atomic bomb survivors in Hiroshima and Nagasaki.
TRUE EXPERIMENTS con’t. - 1· True experiments are more involved and more expensive than observational studies or natural experiments but are still cheaper and easier than laboratory-based research.
· Classical examples of true experiments before World War 2 were not as complicated as today’s experiments but were run on similar basic principles.
TRUE EXPERIMENTS con’t. - 2· 1754 Dr. James Lind published results of his trial of citrus fruit juice to prevent scurvy among sailors. He studied 12 sailors who were given a standard diet. They were allocated to 6 treatment groups that received various treatments for a period of 14 days.
TRUE EXPERIMENTS con’t. - 3· 1886 Cuban epidemiologist Juan Carlos Finlay published his experiments on transmission of yellow fever to humans by the mosquito. He started by recruiting 20 volunteers, 5 were experimental and 15 were controls. He used mosquitoes that had bitten yellow fever to bite his subjects who subsequently developed yellow fever. He continued the experiment with more volunteers.
STRENGTHS OF EXPERIMENTAL STUDIES· 1915 Joseph Goldberger using dietary intervention discovered the dietary cause of pellagra. In 1937 niacin deficiency was identified as the cause of pellagra.
WEAKNESSES OF EXPERIMENTAL STUDIES· The main strength of experimental studies is good control for confounding.
· Randomization in experimental studies is the basis for unequivocal evidence of causality.
· The experimental design enables the investigator to control extraneous variables.
· The experimental design enables the investigator to vary the levels of independent variables in order to make a more thorough and detailed study.
POPULATIONS· The main weakness of experimental studies is that well controlled experiments on humans are difficult to carry out and have ethical problems.
· It is difficult to put humans under full experimental conditions where they can be observed for 24 hours.
· Ethical controversies and violation of human rights always arise in such studies.
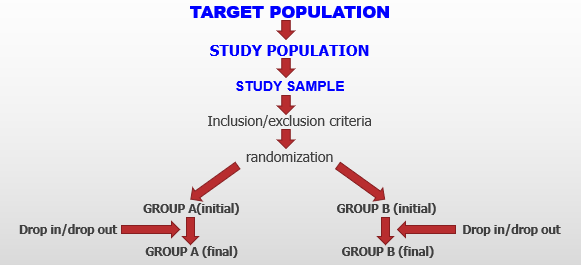
POPULATIONS con’t.· The target population is the big population to which the results of the experiment will be applied.
· Study population: A study population suitable for the problem being studied must be selected. It may represent the general population or may be restricted to certain groups. Economic and logistic considerations affect the selection of the study population.
FIGURE:· Sample are the actual people selected from the study population for the experiment. A representative sample is not necessary for validity of an experimental study.
· Study groups: The researcher determines the experimental or intervention group (who will get the exposure studied) and the comparison group. The control group could get the exposure studied unknown to the investigator. Even after randomization the experimental and comparison groups may not be comparable.
RANDOMIZATION: Definition and Objectives
RANDOMIZATION: Definition and Objectives con’t.· Randomization is the basic sampling scheme for experimental studies and clinical trials. It is the main sampling design used in experimental studies.
· Randomization involves allocation of subjects by independent random choice to 2 or more experimental groups.
· The objective of randomization is to prevent investigator bias in selection and allocation of subjects to the treatment and reference groups, ensure comparability of results from the two groups, and increase confidence in the results of the study.
RANDOMIZATION: Methods· Randomization balances the distribution of confounding as well as prognostic factors and assures accuracy of statistical tests.
· Randomization is not successful with small samples and does not always ensure correct conclusions.
RANDOMIZATION: Enhancements· Alternate cases.
· Sealed serially numbered envelopes.
· Random numbers.
RANDOMIZATION: Parallel and Crossover· Randomization has the objective of balancing the distribution of confounding factors, known and unknown, across groups. This purpose is not always achieved to 100% perfection.
· To obtain more balancing of confounding randomized block design is used in which the randomization process is carried out separately for each 'block'. The block could be a gender or age group.
· Stratified randomization is akin to block design of experimental studies.
RANDOMIZATION: Alternatives· Once the 2 groups are identified, the study may be carried out in parallel or it may use the cross-over design.
· In the parallel design the experimental and comparison groups are followed until the end.
· In the crossover there is an exchange. The experimental group becomes the comparison group during the trial and vice versa. Thus, subjects in both groups will take turns to receive the material being tested.
ETHICAL ISSUES IN CLINICAL TRIALS· Where randomization and selection of a good control group are not possible, quasi-experimental studies may be carried out.
· Non-randomized trials can be carried out where randomization proves difficult, but they are far less valid than the randomized ones. They obtain comparison groups by using either historical controls or by carrying out pre- and post-test assessments.
FURTHER READING· Individual rights/welfare vs community benefit: why should a patient take a risk to discover a new treatment that will benefit future patients.
· Randomization: control/placebo group does not benefit, or the experimental group is exposed to danger.
· Informed consent and coercion in the vulnerable.
· Conflict of interest.
· Confidentiality.
· Publication malpractices: no publication, publication bias.
· The Oxford Textbook of Clinical Research Ethics.