Presentation at the Writing Successful Research Proposal & Manuscript for Health Care Providers held at KFMC, Riyadh on 7-8 April 2019. By Prof. Omar Hasan Kasule Sr. MB ChB (MUK), MPH (Harvard) DrPH (Harvard) Chairman, Institutional Review Board - KFMC
PRIOR PREPARATION:
} Do you have a research question?
} Do you have a clear hypothesis?
} Have you determined the outcome variable(s)?
} Have you determined the determinant variable(s)?
MAIN TYPES OF IRB SUBMISSION:
} Exempt: has no risk to humans mostly interviews,
data from records, or laboratory work.
} Expedited: has minimal risk, the risk is what
is associated with normal outpatient procedures.
} Full review: this has major risk in the form
of intervention by drugs or devices.
REQUIREMENTS FOR EXEMPT SUBMISSION:
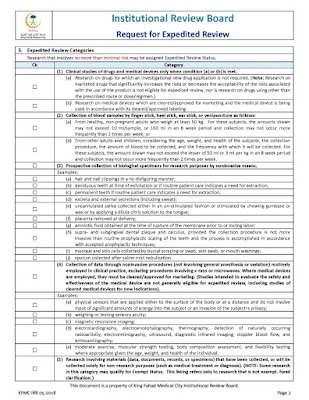
REQUIREMENTS FOR EXPEDITED SUBMISSION:
REQUIREMENTS FOR FULL REVIEW SUBMISSION:
HOW TO SUBMIT:
} All documents submitted electronically.
} The research forms must be signed by the PI
and head of department.
} The proposal must be a separate word document
to enable plagiarism check.
DETAILS OF THE MAIN FORMS:
} The exempt form (see attached).
} The expedited form (see attached).
} The full review form (see attached).
THE EXEMPT FORM:
THE EXPEDITED FORM:
THE FULL REVIEW FORM:
DETAILS OF THE PROPOSAL:
} Title.
} Introduction/background/literature review.
} Aims and objectives.
} Methods.
MALPRACTICES:
} Plagiarism.
} Authorship.